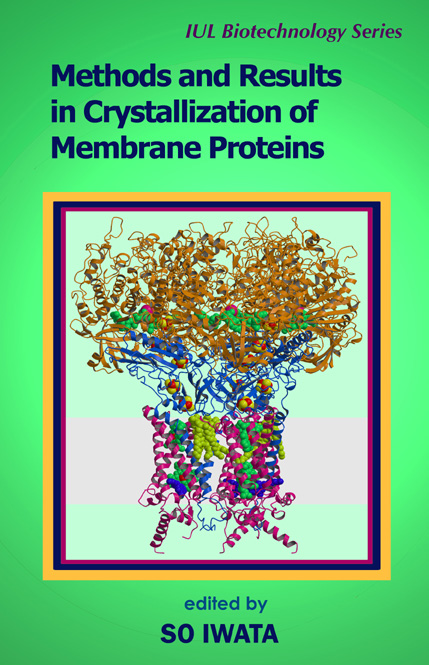
Methods and Results in Crystallization of Membrane Proteins
$109.95
IUL Biotechnology Series, 4
by So Iwata (Editor)
Edition: First
Book Details:
- Series: IUL Biotechnology Series
- Volume: 4
- Binding: Hardcover
- Pages: 375
- Dimensions (in inches): 1.25 x 9.5 x 6.50
- Publisher: International University Line
- Publication Date: June 29, 2003
- ISBN: 0-9636817-9-6
- Price: $109.95
I am often asked by people what the trick is to be so successful in obtaining membrane protein crystals. My answer is always “No trick is the trick”. Nobody has ever succeeded in producing a magic solution for membrane protein crystallisation. Therefore, I do not stick to one particular method but try all possible approaches. This flexibility, together with some confidence, may be keys to success. Unfortunately, this determination itself cannot help you to cover the whole multidimensional space of screening conditions of membrane proteins. Therefore, we need a clear strategy to cover the most relevant conditions as efficiently as possible. I believe that understanding the principles of membrane protein crystallisation and learning from successful examples are the key to more rational and efficient screening. Based on this policy, this book was edited. I collected as many successful examples of membrane protein crystallisation as possible and provided chapters to summarize these results. Also included are chapters dealing with the basic principles of membrane proteins such as phase diagrams and classification of membrane protein crystals. I hope this combination is useful for designing your crystallisation experiment. My last word of advice for people starting membrane protein crystallisation is “Think hard and work hard”. I would thank all the colleagues in my laboratory for their help in editing this book. In particular the scientific contributions and language skills of Dr. Bernadette Byrne have been essential for editing this book. I would also thank all the authors who generously provided their contributions and have been patient during the whole editorial process. Mrs. Terese M. Bergfors at Uppsala University initially recommended me to Dr. Igor Tsigelny at IUL and without them, this book would never be materialized. I must also mentions that all my former supervisors provided me with all the skills necessary to edit this book; they are namely Profs. Takahisa Ohta, Noriyoshi Sakabe, Hartmut Michel, and Janos Hajdu. I would extend my heartfelt gratitude to my wife Momi and my mother Asako for all their support through my entire academic carrier including editing this book. Finally, I dedicate this book to my late father, Isao. So Iwata
METHODS AND RESULTS IN CRYSTALLIZATION OF MEMBRANE PROTEINS
edited by
SO IWATA
Contents
Preface xiii
List of Contributors xv
Part I. INTRODUCTION 1
1. How to Use This Book 3
So Iwata and Bernadette Byrne
1.1. Solubilization and Purification of Membrane Proteins 5
1.2. Crystallization of Membrane Proteins 7
1.3. Detergent Selection 8
1.4. Antibody Approach 9
1.5. Where Do We Start? 9
1.6. Conclusions 10
Part II. PRINCIPLES AND TECHNIQUES IN MEMBRANE PROTEIN CRYSTALLIZATION 13
2. Solubilizing Detergents for Membrane Proteins 15
Melvin H. Keyes, Don N. Gray, Ken E. Kreh, and Charles R. Sanders
2.1. Introduction/Background 17
2.2. Need for Detergents 18
2.3. Requirement for Many and Varied Detergents 19
2.3.1. Different for Each Membrane Protein 19
2.3.2. Sugar-Based Detergents 19
2.3.3. Lipid-Like Detergents 24
2.3.4. Cyclohexyl Alkyl Detergents 26
2.3.5. Polyoxyethylene Detergents 27
2.4. Biochemical Testing of CYFOS™, FOS-MEA™, and FOS-CHOLINE® Detergents 27
2.4.1. Lipid Solubilization 27
2.4.2. Reconstitutive Refolding of a Misfolded Membrane Protein Facilitated by FOS-CHOLINE®, CYFOS™, and
FOS-MEA™ Detergents 28
2.5. Strategies and Criteria for Detergent Selection 30
2.5.1. Extraction 30
2.5.2. Crystallization and Molecular Studies 31
2.5.3. Practical Observation Regarding Detergent Solubility and Purity 31
2.5.4. Price versus Risk 33
3. Crystallization of Membrane Proteins in Lipidic Cubic Phases 39
Ehud M. Landau
3.1. Introduction 41
3.2. Crystallization in Lipidic Cubic Phases 42
3.2.1. Preparing Cubic Phases 42
3.2.2. Preparing Cubic Phases for Crystallization of Membrane Proteins 45
3.2.3. Crystal Handling 50
3.3. Summary 50
4. Practical Aspects of Membrane Protein Crystallization in Lipidic Cubic Phases 57
Peter Nollert
4.1. Operation Principle and General Considerations 59
4.2. Preparation of LCP Crystallization Setups 60
4.2.1. Glass Tubes 60
4.2.2. Preparing the LCP in a Glass Tube 61
4.2.3. Mixing and Dispensing Using Syringes 61
4.2.4. Variable Parameters in LCP Crystallization Screens 63
4.3. Inspection of Crystals Grown in LCP 65
4.3.1. General Remarks 65
4.3.2. Instrumentation for Inspection 66
4.3.3. Colored Protein Crystals 67
4.3.4. Non-Colored Protein Crystals 68
4.3.5. Potential Problem Areas, False Positives 69
4.3.6. Guidelines for Observing LCP Crystallization Setups 70
5. Antibody Fragment-Mediated Crystallization of Integral Membrane Proteins: A Review 73
Christian Ostermeier
5.1. Introduction 75
5.2. Membrane Proteins 76
5.3. How can Membrane Proteins Form Well-Ordered Type II Crystals? 80
5.4. The Critical Role of the Polar Surface 81
5.5. Enlargement of the Polar Surface Area 81
5.6. Antibody Fragments 82
5.7. Production of FV Fragments 83
5.7.1. Cloning 83
5.7.2. Expression 83
5.8. FV Fragments as a Tool for Isolation and Crystallization 84
5.8.1. FV Fragments as a Tool for Purification 84
5.8.2. Conformational Epitopes 84
5.8.3. FV Fragments as a Tool for Crystallization: They Really Work! 85
5.9. Alternatives to FV Fragments 86
6. Generating Antibody Fragments for Structural Studies: A Guide 89
Bernadette Byrne and So Iwata
6.1. Introduction 91
6.2. Hybridoma Cell Line Production 92
6.3. Procedure 93
6.3.1. Immunization 93
6.3.2. Fusion 93
6.3.3. Screening of Hybridoma Cell Lines 94
6.4. Fab Fragments 95
6.5. FV Fragments 97
6.6. Summary 98
7. Crystallization of Bacterial Outer Membrane Proteins from Detergent Solutions: Porin as a Model 101
Gabriele Rummel and Jurg P. Rosenbusch
7.1. Stating the Problems 103
7.2. Considering Potential Remedies 104
7.3. Porin as a Model 106
7.4. Criteria for Rational Detergent Selection 106
7.5. Polymorphism of Detergents and Membrane Proteins in Solution: The Significance of the Phase Diagram 114
7.6. Crystallization of Porin and Other Bacterial Outer Membrane Proteins: A Brief Survey 118
7.7. Monodispersity of Building Blocks versus Micelle Dynamics: Lessons from Protein-Detergent Contacts in Crystals 118
7.8. Porin as a Paradigm: Is It a Valid Model? 121
7.9. Conclusions and Perspectives 123
8. Crystallization of Membrane Proteins in Oils 131
Naomi E. Chayen
8.1. Introduction 133
8.2. The Automated Microbatch Technique 134
8.3. Examples of Membrane Proteins Crystallized under Oil 134
8.4. Advantages of Crystallization in Oils 135
8.5. Harvesting the Crystals 136
8.6. Summary and Future Developments 137
8.7. Exercises 137
8.7.1. Materials Required 137
8.7.2. Screening Procedure 138
8.7.3. Optimization 138
PART III. EXAMPLES OF SUCCESSFUL CRYSTALLIZATION OF MEMBRANE PROTEINS 141
A. Complexes in Photosynthesis 143
9. Crystallization of Photosystem I 145
Petra Fromme
9.1. Introduction 147
9.2. Results and Discussion 148
9.3. Biological and Biochemical Parameters 149
9.3.1. The Organism 149
9.3.2. The Physiological Status of the Organism 150
9.3.3. The Quaternary Structure and the Subunit Composition 151
9.3.4. Proteolytic Digestion and Ageing of the Protein 153
9.3.5. Binding of “Ligands” 153
9.4. Physical and Chemical Parameters 155
9.4.1. Ionic Strength 155
9.4.2. Nature of Salts 159
9.4.3. pH 160
9.4.4. Temperature 161
9.4.5. Detergent 162
9.4.6. Crystallization Agents 166
9.4.7. Diffusion and Convection (Gravity/Microgravity) 167
9.5. Nucleation and Seeding Techniques—Micro- and Macroseeding 168
B. Respiratory Complexes 175
10. Crystallization of Wolinella succinogenes Quinol:Fumarate Reductase in Three Crystal Forms 177
C. Roy D. Lancaster
10.1. Introduction 179
10.2. Preparatory Steps 181
10.2.1. Growth of Wolinella succinogenes 181
10.2.2. Isolation of Quinol:Fumarate Reductase 182
10.3. Crystallization of Quinol:Fumarate Reductase 183
10.4. Characterization of the Crystals 185
10.5. Structure Determination 187
11. Crystallization of the Respiratory Complex Formate Dehydrogenase-N from Escherichia coli 193
Mika Jormakka
11.1. Introduction 195
11.2. Expression and Purification of Fdn-N 196
11.3. Crystallization and X-ray Data Collection of Fdn-N 196
11.4. Structure Determination of Native Fdh-N 198
11.5. Determination of the Quinone Binding Site 200
12. Crystallization of the Cytochrome bc1 Complex 203
Li-Shar Huang, David Cobessi, and Edward A. Berry
12.1. About the Protein 205
12.2. Purification Procedures 206
12.3. Early bc1 and b6f Crystallization Studies 207
12.4. Three-Dimensional Crystals of Mitochondrial Cytochrome bc1 208
12.5. Large Tetragonal Crystals from the Yu Preparation 209
12.6. Monoclinic, Tetragonal, and Hexagonal Crystals from the Rieske Preparation 210
12.7. Crystallization of the bc1 Complex from Various Vertebrate Organisms in Berkeley 211
12.8. Improved Beef bc1 Crystals from the Jap Group at Berkeley and the Iwata Group in Uppsala 213
12.9. Higher Resolution Crystals of the Fungal bc1 Complex from MPI-Frankfurt 213
12.10. Unpublished Observations from the Berkeley Group 214
12.10.1. Needle Crystals of the Bovine bc1 Complex 214
12.10.2. Hexagonal Bipyramid Crystals (P6522) 215
12.10.3. Conditions for Growth 216
12.10.4. Hexagonal Bipyramid Crystals without Seeding 217
12.10.5. Precrystallization 218
12.10.6. Rabbit Cytochrome bc1 Crystals 218
12.10.7. Orthorhombic Chicken bc1 Crystals 219
12.11. Conclusions 221
13. Crystallization of Cytochrome bo3 Ubiquinol Oxidase from E.coli 227
Jeff Abramson, Bernadette Byrne, and So Iwata
13.1. Purification, Crystallization, and X-Ray Data Collection for Cytochrome bo3 229
13.1.1. Crystal Form 1: Wild-Type Cytochrome bo3 229
13.1.2. Crystal Form 2: Cytochrome bo3-Fusion Complex 233
13.2. Structure Determination of Cytochrome bo3 235
13.2.1. Structure Determination for Crystal Form 1: Wild-Type Cytochrome bo3 235
13.2.2. Structure Determination and Interpretation for Crystal Form 2: Cytochrome bo3-Protein Z Fusion 236
C. Channel and Receptor 239
14. Crystallization and Structure Determination of MscL, a Gated Prokaryotic Mechanosensitive Channel 241
R. H. Spencer, G. Chang, R. B. Bass, and D. C. Rees
14.1. Introduction 243
14.2. Target Identification 244
14.3. Protein Expression 245
14.4. Protein Purification 246
14.5. Protein Crystallization 248
14.6. Crystallographic Analysis 248
14.7. Conclusions 250
15. Crystallization of Bovine Rhodopsin, a G Protein-Coupled Receptor 253
Tetsuji Okada
15.1. Introduction 255
15.2. Purification Procedure 256
15.2.1. Membrane Preparation 256
15.2.2. Selective Solubilization 257
15.3. Detail of the Crystallization Experiments 258
15.3.1. Protocol 258
15.3.2. The Case History 259
15.4. Characterization of the Crystals 260
15.5. Summary of the Structural Determination 261
15.6. Remarks 261
D. Outer Membrane Proteins 263
16. Crystallization of Phopholipase A in Two Biological Oligomerization States 265
Arjan Snijder, Thomas Barends, and Bauke W. Dijkstra
16.1. Introduction 267
16.2. Purification 268
16.3. Crystallization 269
16.3.1. Monomeric OMPLA 269
16.3.2. Crystal Packing of Monomeric OMPLA 271
16.3.3. Dimeric OMPLA 273
16.3.4. Crystallization under Oil 274
16.3.5. Crystal Packing of Dimeric OMPLA 279
16.4. Conclusion and General Message 276
Part IV. CRYSTALLIZATION INFORMATICS OF MEMBRANE PROTEINS 279
17. Crystallization Informatics of Membrane Proteins 281
So Iwata
17.1. Introduction 283
17.2. Design of a Kit for Membrane Protein Crystallization 284
17.2.1. Selection of Precipitants 286
17.2.2. Selection of Buffers 288
17.2.3. Selection of Salts 288
17.2.4. Design of the Screening Kit 291
17.3. User Instruction of the Kit 291
17.3.1. Protein Concentration 292
17.3.2. Selection of Detergent 292
17.3.3. Sample Buffer 293
17.3.4. Temperature 294
17.3.5. Additives 294
17.3.6. Observation 294
17.4. Summary 294
APPENDICES 299
Questionnaire 302
A1. Porin (OmpF) from Escherichia coli 304
A2. Porin from Rhodobacter capsulatus 305
A3. Porin from Rhodopseudomonas blastica 306
A4. Porin from Paracoccus denitrificans 307
A5. Porin Omp32 from Comamonas acidovorans 308
A6. Phosphoporin from Escherichia coli 309
A7. Osmoporin from Escherichia coli 310
A8. Osmoporin from Klebsiella pneumoniae 311
A9. Maltoporin from Escherichia coli 312
A10. Maltoporin from Salmonella typhimurium 313
A11. Sucroseporin from Salmonella typhimurium 314
A12. Exoporin from Escherichia coli 315
A13. Siderophore translocator (FhuA) from Escherichia coli 316
A14. Siderophore translocator (FhuA) from Escherichia coli 317
A15. Siderophore translocator (FepA) from Escherichia coli 318
A16. Truncated transmembrane domain of OmpA-protein from Escherichia coli 319
A17. OmpX-protein from Escherichia coli 320
A18. Phospholipase A from Escherichia coli outer membrane, monomer 321
A19. Phospholipase A from Escherichia coli outer membrane, dimer 322
A20. MscL from Escherichia coli 323
A21. Photosystem I 324
A22. Cytochrome c oxidase from Paracoccus denitrificans 325
A23. Cytochrome bc1 complex, P65 form 326
A24. Cytochrome bc1 complex, P6522 form 327
A25. Ubiquinol oxidase from Escherichia coli 328
A26. Formate dehydrogenase from Escherichia coli 329
A27. Succinate dehydrogenase from Escherichia coli 330
A28. Cytochrome c oxidase from Rhodobacter sphaeroides 331
A29. Halorhodopsin 332
A30. Bacteriorhodopsin 333
Index 339
Preface
Acknowledgements
London










H. Ronald Kaback, M.D. –
Howard Hughes Medical Institute Investigator, Professor, University of California, Los Angeles
As a scientist working in the field of membranes and membrane proteins who has tried to crystallize a membrane transport protein for over a decade, I may be somewhat prejudiced. However, I feel that membrane protein structure/function is the field of the future in biochemistry and physiology. The number of high-resolution structures of membrane proteins currently available is infinitesimal relative to the number of structures available for soluble proteins, yet the two most widely sold pharmaceutical agents in the world are targeted to membrane proteins (Prozac and Imiprazole), and membrane proteins play important roles in the pathophysiology of numerous human diseases (e. g., Cystic Fibrosis). Although the number of high-resolution membrane protein structures has begun to increase within the past few years, I know of no treatise that deals comprehensively with the practical problems inherent in crystallization of this class of protein.
Iwata’s book represents just such a treatise. Parts I and II deal with general principles and techniques in membrane protein crystallization in a clear and concise fashion, with an excellent general introduction followed by chapters covering the use of detergents, crystallization in lipidic cubic phases, the use and generation of antibody fragments, porin as a model and crystallization of membrane proteins in oils. Part III focuses on specific examples of membrane proteins whose structures have been solved. The examples cover the gamut of membrane proteins from those involved in photosynthesis to respiratory complexes to channels, receptors and the outer membrane protein phospholipase A. In each case, the chapters are written in a lucid and practically oriented fashion by the very scientists who solved the structures. Finally, in Part IV, Iwata provides a particularly useful practical guide to begin crystallization of a-helical membrane proteins.
This is an superb, timely book that will be of great interest and practical use to both students and practitioners of membrane protein crystallization.
Richard Cogdell –
Professor, University of Glasgow
Determination of the structure of membrane proteins remains a major challenge in structural biology. One of the biggest hurdles to overcome is to produce well-ordered 3D crystals of membrane proteins that are suitable for X-ray crystallography. This excellent book, edited by So Iwata, provides a case-by-case guide of successful strategies that have been used to tackle this problem. The text provides the theory of how membrane proteins crystallize and then illustrates this with real examples. It will become a ‘must read’, for any scientists wishing to move into this exciting research area and give them the confidence to succeed!